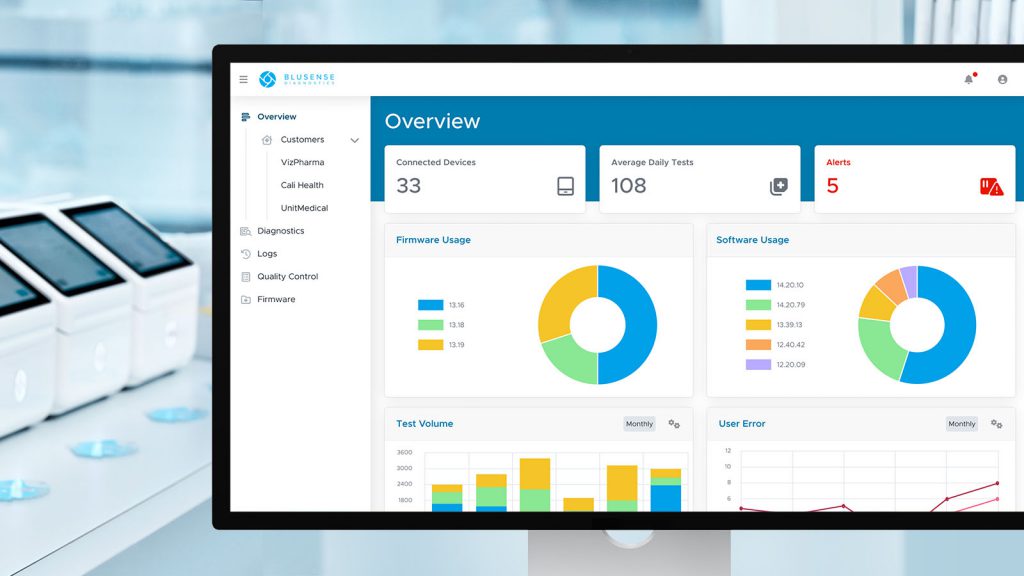
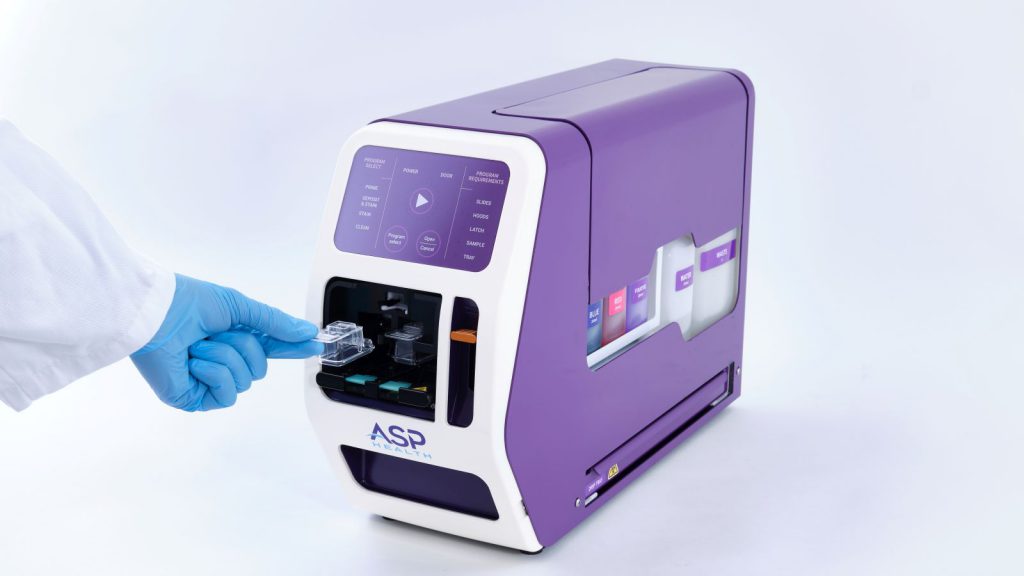
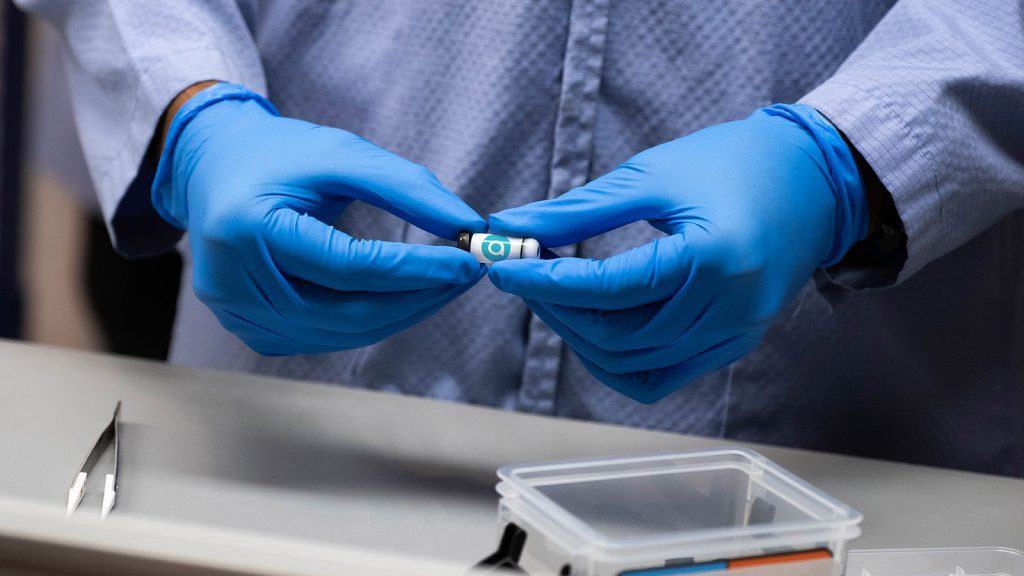
Truvian Health raises $74M - in part to secure FDA clearance for its blood testing platform
Truvian Health recently announced an injection of USD$74 million of capital to be used in part to secure US Food and Drug Administration (FDA) clearance for its blood testing platform.