As demand for diagnostic and point-of-care (POC) testing grows, new products are coming to life faster than ever. I recently had the pleasure of moderating a panel at the MD&M and BIOMEDigital event, where I got to discuss the challenges and opportunities for developing POC diagnostic products in a post-COVID world. Joining me on the panel were Anthony Favaloro, Director of Strategic Partnerships at Lumos Diagnostics, Ragu Raman, President and CEO of Integrated Nano-Technologies, and Natalie Kennel, founder of regulatory consultancy NJK & Associates.
Here are my key takeouts from the session.
Accelerated product development timelines makes it critical to get key roles in place early
The urgent need for COVID testing products meant that teams that would normally take two to three years to develop a product were being asked to turn them around in a year, and year-long projects were being turned around in just three months. With these accelerated timelines, it’s crucial to get key people in key roles early.
As Anthony said: “Roles in asset development, design transfer, quality and regulatory, usability – we really need to get those roles in place and communicating cross-functionally from the very beginning to ensure that everybody knows what they need to be doing and we’re being as efficient as possible.”
Don’t let speed outweigh quality
While time to market is key, this should not come at the cost of quality or your unique differentiator. This is particularly true if you’re a start-up. Ragu, who has recently moved from working at a large organization to working at a start-up, said there was a greater need for start-ups to get their product right first time.
“In a unique situation like the pandemic, the immediate reaction is let’s find a way to make a product come together in a short time. The larger organizations can go with the first version and then have a second bite of the cherry. But if you’re a start-up company, you have to establish credibility with your customers, your regulators, your investors, etc., so you have to be very thoughtful about the push for speed and make sure that you don’t lose your unique differentiation and unique value,” Ragu said.
Natalie added that when it comes to development of regulated products, sometimes slower could be faster.
“It’s the tortoise and the hare, where sometimes it’s better to go a little slower and more methodically and make sure it’s done right the first time, rather than rushing and having to redo it five times,” Natalie said.
Start-ups need to develop strong partnerships
Start-ups tend to have fewer decision-makers and leaner processes, which makes it easier for them quickly respond to new needs, such as for COVID testing. The flipside is that this leanness means that staff tend to be hired for their versatility and breadth of knowledge – which can lead to a lack of depth of expertise. This can be addressed by forming partnerships with other organizations or suppliers that have the skills you need.
As Ragu said: “Institutional rigor comes naturally in large organizations. Quality, regulatory, usability, serviceability, etc. – all of the mechanics of a full function product and making sure that it is a strong solution, I think that naturally comes in a large organization because the people and processes are already in place.
“For a start-up, that’s a little bit more of a challenge. So that’s where having access to the right partners and cultivating a partner landscape becomes really important, from a regulatory standpoint, for industrial design, for digital infrastructure, etc.”
Point-of-care test needs to be accurate and reliable
COVID has accelerated a move towards point-of-care testing that was already underway as people take more responsibility for their health and wellbeing. People want to be able to get tested at a location convenient to them, and they want to get their results back as soon as possible. That’s not to say that lab-based tests are going to go away, but there’s going to be a lot more emphasis on how we can move tests from the lab to the clinic and the clinic to the home.
However, for point-of-care tests to really take off, they need to have comparable accuracy and reliability to that of lab-based tests. Natalie said the trade-off between performance and convenience had been one of the challenges of the pandemic.
“A lot of the antigen tests just weren’t quite good enough. And there were a lot of technical debates about how good is good enough in this situation. But the fact of the matter is that they’re not as sensitive in general as the PCR tests, but they’re a heck of a lot quicker to use, so there’s a trade-off there,” Natalie said.
“But I see that bar moving up and I see there are several examples of PCR-based or molecular-based tests that have been moved to the point-of-care and are pretty good. So that’s a trend that’s going to continue, pandemic or not.”
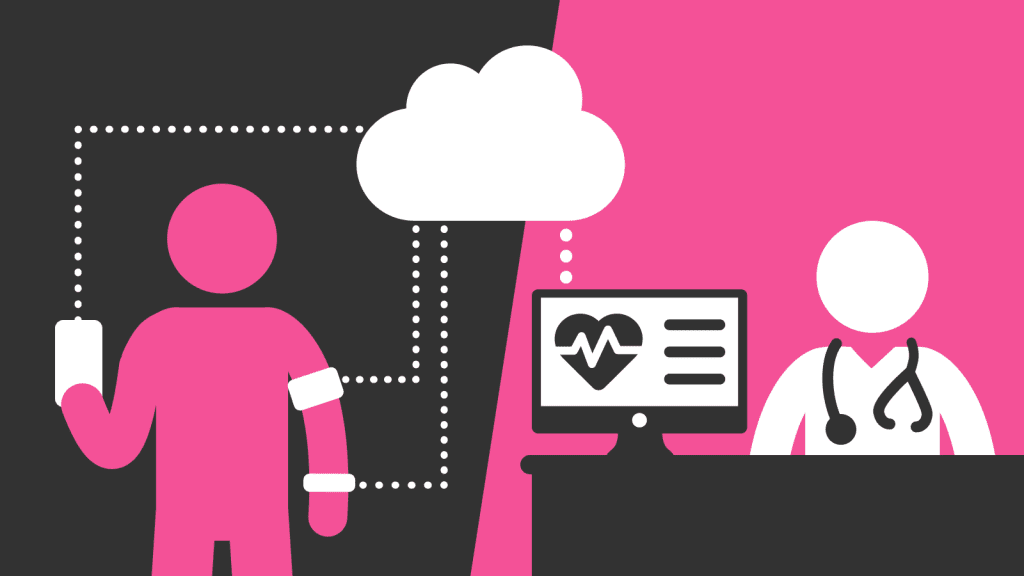
Build in digitization and connectivity
The point-of-care testing space is moving toward greater digitization. At the first level, this means more digitally displayed results to take the subjectivity out of visually read tests and make them easier to use. At the next level, this means greater connectivity – with a trend toward smartphone apps and cloud integrations. Data collection, while bringing some privacy challenges, can lead to integration with Electronic Health Records, better disease tracking, and potentially AI-generated analysis.
“There is so much data being collected now, and obviously there’s a certain degree of analysis and processing that needs to be done on the data, but the opportunities are really becoming endless from a health and wellbeing perspective,” Anthony said.
The user experience should be simple and seamless
The final takeout is one close to my heart as a designer – user experience. Users are getting a lot more discerning about their products. They expect a good user experience from sample to result. This becomes even more important in the point-of-care space, when your users may be minimally trained staff in clinics or pharmacies, or even the consumer themselves. If you are producing a CLIA-waived product, you need to make sure your user experience is so simple and intuitive that most people could follow it just by reading basic instructions. For products targeted at trained clinicians, you need to consider their workflows and busy schedules. Your product needs to make their lives easier, not add another layer of work. The simpler and more seamless you can make your user experience, the more your product will be adopted.
Final thoughts
The last 12 months have focused the world’s attention on diagnostic testing and really accelerated a trend towards point-of-care. So many innovative products have already hit the market and I know we will see many, many more in the years to come. COVID has really lit a fire underneath the point-of-care diagnostic rocket, leading to more choice, greater convenience and hopefully better health outcomes.