Capabilities
PI has the full spectrum of product design, engineering and manufacturing capabilities needed to develop and commercialize products for the diagnostics, life sciences, cell and gene therapy, and healthcare industries.
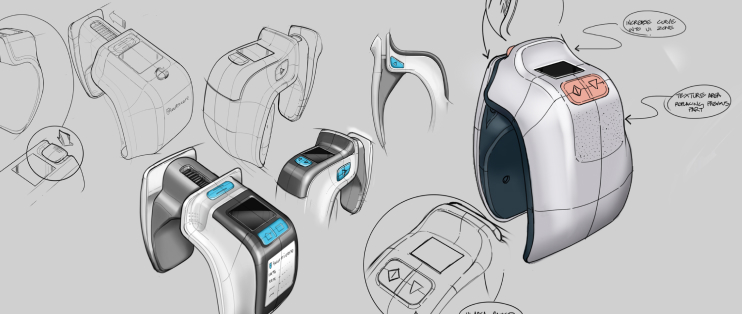
We take a user-centric and market-focused approach to industrial design, ensuring your products are not only beautiful, but also user-friendly, practical and cost-effective.
The mechanical design team draws on decades of expertise in medtech and other regulated industries to guide the realization of complex and novel products, leveraging world-class tools, proven manufacturing processes and PI’s proprietary innovation methods to deliver commercial success.
PI’s electronic engineering team is highly capable in the design of complex electronics for medical devices including: system architecture, schematic & PCB layout using Altium designer, loom design, prototyping, assembly and transfer to manufacture.
The systems team are experienced product developers that specialize in defining a product vision and taking a device on a journey to realize that vision. Our involvement begins at the early stages of product definition and architecture refinement, and continues throughout the development, monitoring risks, performing testing and managing verification activities.
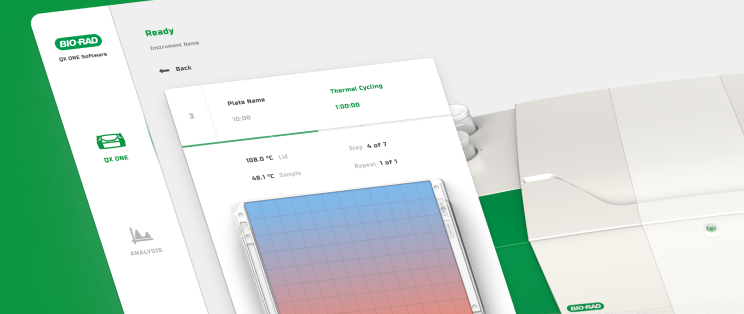
We create tailored software solutions for regulated medical environments, leveraging extensive experience in product development and market success. Our team spans the full breadth of software talent (developers, testers, and architects) offering development for a wide range of technological needs, including PC applications, firmware, mobile apps, and cloud.
Our UI/UX team is experienced in designing user interfaces for a range of applications including device embedded screens, mobile and web apps, and wearables.
Our Human Factors & Usability Engineering team humanizes medical technologies through qualified and expert understanding of people their behaviours and their limitations. We guide design direction – providing insights, backed up by data, on how your system will be used, perform and be safe.
Planet Innovation offers targeted optics support for your product, with a dedicated team of optics and opto-mechanical specialists who have demonstrated capability in developing and manufacturing integrated optical systems for medical instruments.
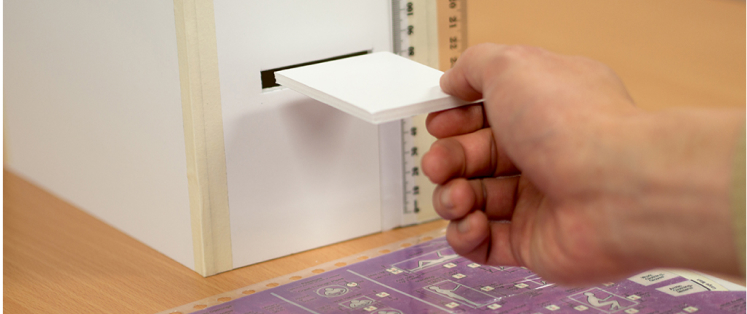
Planet Innovation has onsite labs and scientists with broad experience across medical device development.
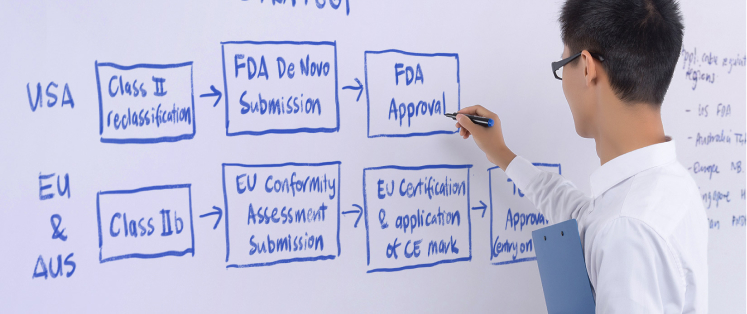
PI’s quality team are experienced in quality design assurance for regulated and non-regulated medical devices. Our robust quality management system is certified to ISO 13485:2016. We also have regulatory experts who can provide tailored regulatory advice for developing medical products.
Our New Product Introduction team are experts on how to manufacture high quality, regulated healthtech products so they can be brought to market quickly and cost effectively.
At our FDA registered and ISO 13485:2016 compliant manufacturing facilities, we can produce anything from low-complexity devices to large floor-standing in vitro and clinical chemistry instruments, in quantities ranging from small batches to high-volume builds.
We have an ISO 7 certified on-site cleanroom to manufacture consumables. We are experienced in processes including solvent bonding, ultrasonic welding, leak testing, air particle testing, heat sealing, gamma sterilization, bioburden and sterility testing.
PI produces the highest quality products that adhere to FDA regulations, crafted within a well-established quality system that undergoes continuous auditing. These processes take place in certified facilities that meet the standards of ISO 13485:2016 and ISO 9001.
PI has experts across all aspects of product commercialization from initial product definition to achieving market success. We start with in-depth market analysis and MVP definition, then follow through with actionable plans covering regulatory compliance, clinical development, marketing and product launch.
Our Project Management team is focused on guiding our teams to deliver high quality project outputs that achieve our clients’ individual goals. We bring a specialized understanding of the product development nuances for the regulated medical industry.
PI’s BRIGHT process is our innovation framework that underpins all of our product development and commercialization activities.
Contact Us
Learn more about our capabilities.
PI offers the full spectrum of product development and manufacturing capabilities needed to create and commercialize sophisticated, connected products. We can help you with any or all of your needs – from identifying a market opportunity, through design, engineering and digital, to manufacture and commercial launch. Our flexible approach and ability to scale support means we can tailor our services to suit your needs
Let’s talk