Project Management
Delivering collaborative projects that achieve our client’s individual goals.
Our Project Management team is focused on guiding our teams to deliver high quality project outputs and providing commercial program guidance. We bring multi-disciplinary teams together across the globe to achieve common development goals in highly regulated industries. We prototype early and utilize experts (both in-house and client) to build and test sophisticated product systems.
Project delivery
Ensuring the project outputs are delivered to strategic timelines and costs are controlled
Medical focus
Specialized understanding of the product development nuances for the medical industry
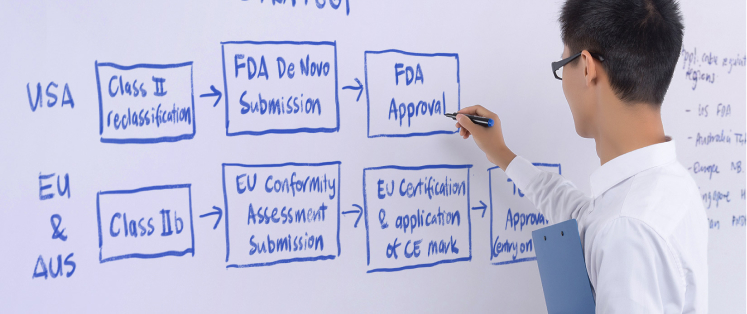
Regulatory expertise
Inhouse Quality and Regulatory Assurance teams define appropriate strategies
Agile approach
Building fast and building lean prototypes to test the risks and learn quickly
Product Development Process
PI’s Product Development Process (PDP) follows the fundamental FDA and ISO 13485 phase gated process and is committed to quality and regulatory compliance. We tailor each program from development through to manufacturing for every client to ensure the right product is developed to meet your individual business case and commercial objectives.


Strategic Focus
Our development process addresses the risks head on and early, prototyping and testing out the technical risks, while reviewing and future planning for project and commercial risks. We provide high level management involvement in your product’s steering committee for strategic collaboration and planning, and to support your path to market. This clear vision allows the team to focus on critical tasks and push to meet key timelines.
Collaborative Teams
We promote a partnership mindset with our clients by offering a blended team approach, embedding a client’s expertise and existing staff into the project to supercharge task completion.
Having PI staff across our Australian and U.S. offices enables us to support multiple time zones, offering onsite client support and efficient cross-team workflows.


Manufacturing Expertise
We incorporate our production engineers into the early design phases of development to ensure the product you verify becomes the product you manufacture, whether at our manufacturing facilities or your own. The close working relationship of our design and manufacturing teams enhances the visibility, communication and knowledge transfer, making manufacturing establishment and production an integrated and smoother process. Our phase gate process incorporates key manufacturing readiness activities throughout to ensure that your manufactured product meets your commercial and regulatory requirements.
Effective Workflows and Communication
We know effective communication is vital to project success. That’s why we prioritize communication between key stakeholders to discuss progress, issues or risks. Regular touchpoints are planned, and channels are mapped for escalating issues and getting fast decisions from product owners, technical leads and others. In addition to regular face-to-face meetings, we often have an operational consultant embedded onsite during development. Having these communication systems in place helps ensure that projects stay on track, within scope, and with the right overall outcome.
We use a number of streamlined online tools to support efficient project and team management, covering:
- Project Management planning, estimating, tracking and reports
- Task and Issue Management
- Device Configuration Management (Prototypes and Production)

Related Capabilities
PI has experts across all aspects of product commercialization from initial product definition to achieving market success. We start with in-depth market analysis and MVP definition, then follow through with actionable plans covering regulatory compliance, clinical development, marketing and product launch.
Our New Product Introduction team are experts on how to manufacture high quality, regulated healthtech products so they can be brought to market quickly and cost effectively.
PI’s quality team are experienced in quality design assurance for regulated and non-regulated medical devices. Our robust quality management system is certified to ISO 13485:2016. We also have regulatory experts who can provide tailored regulatory advice for developing medical products.