November 18-24 is World Antibiotic Awareness Week, so it’s a good time to draw attention to the global health challenge of antibiotic resistance, which Planet Innovation is combating through its business Lumos Diagnostics.
Lumos Diagnostics was spun out of Planet Innovation in 2017 and this year launched the FebriDx test, which is currently approved for use in Europe, Canada, Australia and various other countries.
The FebriDx test allows health professionals to run an all-in-one fingerstick test at the point of care to determine if a patient is suffering from a viral or bacterial infection. This means that antibiotics – which are effective for bacterial infections but of no use for viruses – are only prescribed when genuinely needed. By avoiding unnecessary prescription of antibiotics, we are helping to tackle antibiotic resistance.
Antibiotic resistance is a global health problem
Antibiotic resistance is one of the biggest threats to global health today, occurring when bacteria mutate to become resistant to antibiotics. These strains of antibiotic resistant bacteria are commonly known as ‘superbugs’. According to the World Health Organization, without urgent action, we’re heading for a post-antibiotic era, in which common infections and minor injuries can once again kill.1
Antibiotic resistance is caused by overuse of antibiotics
Unnecessary use of antibiotics increases antibiotic resistance, causing more than 25,000 deaths in the EU, and results in over $20 billion of direct healthcare costs in the U.S. annually.2
One of the leading causes of over-prescription of antibiotics is doctors prescribing antibiotics to patients presenting with cold or flu symptoms. In 90% of cases, patients who present with the most common symptom – an acute cough – have an illness caused by a virus3,4 , which cannot be treated by antibiotics. Despite this, doctors still often prescribe antibiotics to treat patients with cold and flu symptoms, leading to up to 50% of antibiotics being prescribed unnecessarily5.
Why do doctors overprescribe antibiotics?6
There are several factors that contribute to physicians, commonly known as GPs in the UK and Australia, overprescribing antibiotics. Firstly, in the early stages of an illness, the symptoms for viral and bacterial infections are very similar (e.g. a cough), so it can be difficult to tell whether it is a viral infection (i.e. cold or flu), or a bacterial infection. Doctors may prescribe antibiotics to minimize the risk. Another factor is time pressure. Doctors can be under time pressure to see a large number of patients and providing an antibiotic script may be a faster way to complete a consultation rather than explain why an antibiotic is unnecessary. Patients also often request antibiotics, and a doctor may believe that refusing the request could threaten the doctor-patient relationship.
Up until now, there has been no rapid point-of-care test that could tell viral from bacterial infections, meaning doctors had to rely primarily on their clinical judgment to make a diagnosis.
Introducing FebriDx
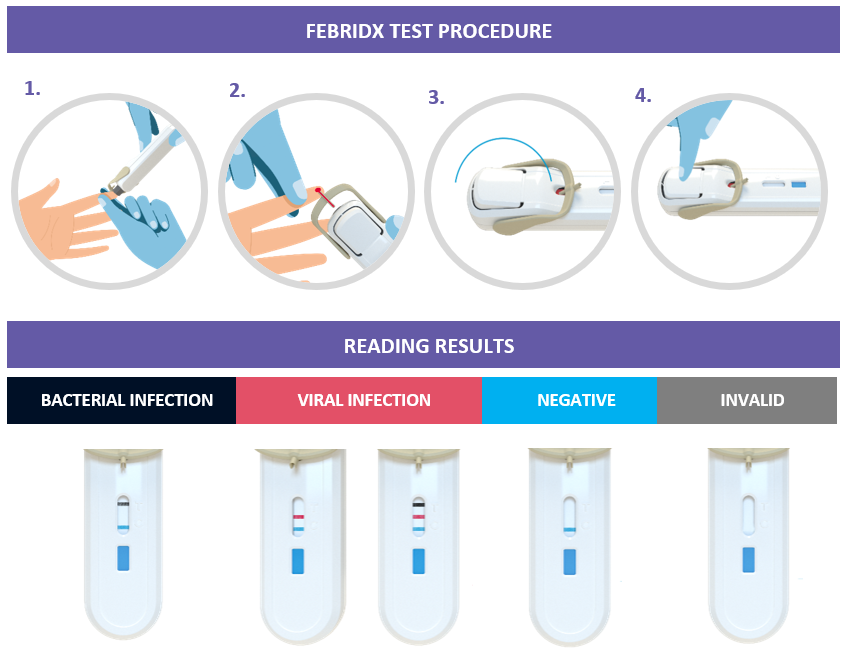
FebriDx is the first and only rapid, all-in-one point-of-care test device that can identify a clinically significant acute respiratory infection (ARI) and differentiate viral from bacterial causes. FebriDx can be used to help triage patients at the point of care to reduce uncertainty and avoid unnecessary antibiotics. The first-generation visually-read FebriDx test is CE-marked and commercially available to health professionals in Europe, UK, Canada and Australia, as well as various other countries.
How it works
FebriDx is an all-in-one test that enables clinicians to take a small fingerstick blood sample (5ul) and get a visually-read result, all within one hand-held device. The test simultaneously detects Myxovirus resistance protein A (MxA) and C-reactive protein (CRP) directly from peripheral whole blood. MxA is an intracellular protein that becomes elevated only in the presence of acute viral infection and CRP is an acute-phase inflammatory protein that is elevated in the presence of clinically significant infection. By simultaneously detecting both CRP and MxA in a single multiplex assay, FebriDx optimizes the sensitivity and specificity of both tests to differentiate between bacterial and viral infections.
Supported by science
Clinical performance from two prospective multi-center US clinical trials demonstrate the FebriDx test’s high accuracy and 97-99% negative predictive value to exclude a bacterial infection.7,8
In a United Kingdom outcome study, FebriDx was shown to alter clinical management decisions in 48% of patients tested and reduced unnecessary antibiotic prescriptions by 80%.9 There are a further 10 clinical studies, involving more than 1,600 patients, either underway or due to start this year in different settings including urgent care, general practice, and emergency departments.
Find out more about FebriDx on the FebriDx website.
References
- ‘Antibiotic resistance’, World Health Organization, 5 Feb 2018 https://www.who.int/en/news-room/fact-sheets/detail/antibiotic-resistance
- EU action on antimicrobial resistance. https://ec.europa.eu/health/amr/antimicrobial-resistance_en. Accessed May 6, 2019.
- Bisno AL. Acute pharyngitis: etiology and diagnosis. Pediatrics. 1996;97(6 pt 2):949–954.
- Strauss RA. Treatment of postviral nonasthmatic cough with corticosteroids. J Allergy Clin Immunol: In Practice. 2013;1:404-6.
- Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults. Ann Intern Med. 2016;165(9):674.
- ‘Nudge vs Superbugs: A behavioural economics trial to reduce the overprescribing of antibiotics’, Australian Government Department of Health and Department of the Prime Minister and Cabinet, June 2018.
- Shapiro, N.I., Self, W.H., Rosen, J., Sharp, S.C., Filbin, M.R., Hou, P.C., Parekh, A.D., Kurz, M.C. and Sambursky, R. ‘A prospective, multi-centre US clinical trial to determine accuracy of FebriDx point-of-care testing for acute upper respiratory infections with and without a confirmed fever’, Annals of Medicine, 18 May 2018. https://doi.org/10.1080/07853890.2018.1474002
- Self, W.H., Rosen, J., Sharp S.C., Filbin, M.R., Hou, P.C. et al. ‘Diagnostic Accuracy of FebriDx: A Rapid Test to Detect Immune Responses to Viral and Bacterial Upper Respiratory Infections’, J. Clin. Med. vol 6, iss. 94, 2017
- Davidson, M. ‘FebriDx point-of-care testing to guide antibiotic therapy for acute respiratory tract infection in UK primary care: a retrospective outcome analysis’, Journal of Infectious Diseases & Preventative Medicine, vol. 5, issue 3, 24 Aug 2017. https://doi.org/10.4172/2329-8731.1000165