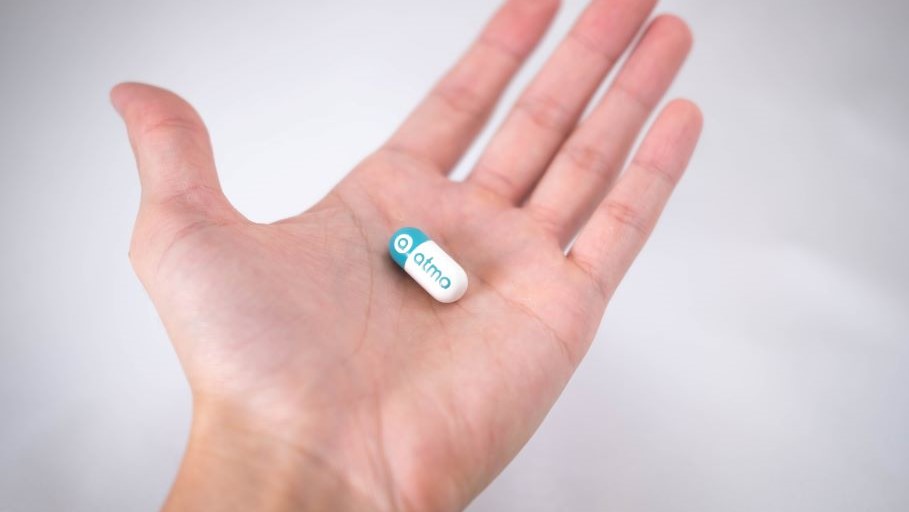
Atmo Biosciences, a digital health business that provides objective real-time insights into gut health and microbiome function, has raised a further A$2.5 million in an oversubscribed funding round, supplementing an initial seed raise in March 2019.
Atmo, one of a portfolio of businesses incubated by Planet Innovation, is underpinned by a world-first ingestible gas-sensing capsule that continuously measures clinically important gaseous biomarkers produced by the microbiome in the gastrointestinal system. This data is transmitted wirelessly to the cloud for aggregation and analysis.
Atmo addresses the unmet clinical need to monitor microbiome function, allowing better diagnosis and management of personalized therapies for gastrointestinal disorders, resulting in improved gut health and wellness. The technology also provides researchers with a platform to evaluate the benefits of probiotics, therapeutic candidates, and functional foods.
Functional insight into the microbiome is the focus of significant medical research efforts globally, as it is increasingly seen as important to understanding a range of conditions. However, the lack of useful tools available to understand microbiome function is holding back knowledge of microbiome impact on human health and the development of personalized therapeutic approaches.
Atmo CEO Mal Hebblewhite said: “One in five people worldwide suffer from a gastrointestinal disorder in their lifetime, and almost a third of these cases remain unresolved, resulting in costs to the healthcare system, decreased productivity, and reduced quality of life.”
“Our aim is for the Atmo Gas Capsule to become the standard for screening and diagnosis of common GI dysfunction and disease, and monitoring of gut health; resulting in better targeted therapy, earlier relief of symptoms, and healthcare cost savings.”
Atmo will use the funds for continued product development, manufacture of the second generation gas-sensing capsule, and pilot clinical trials aimed at developing a path to regulatory approval.
The first of a range of human clinical trials is due to restart in June, after being put on hold due to COVID-19 restrictions.