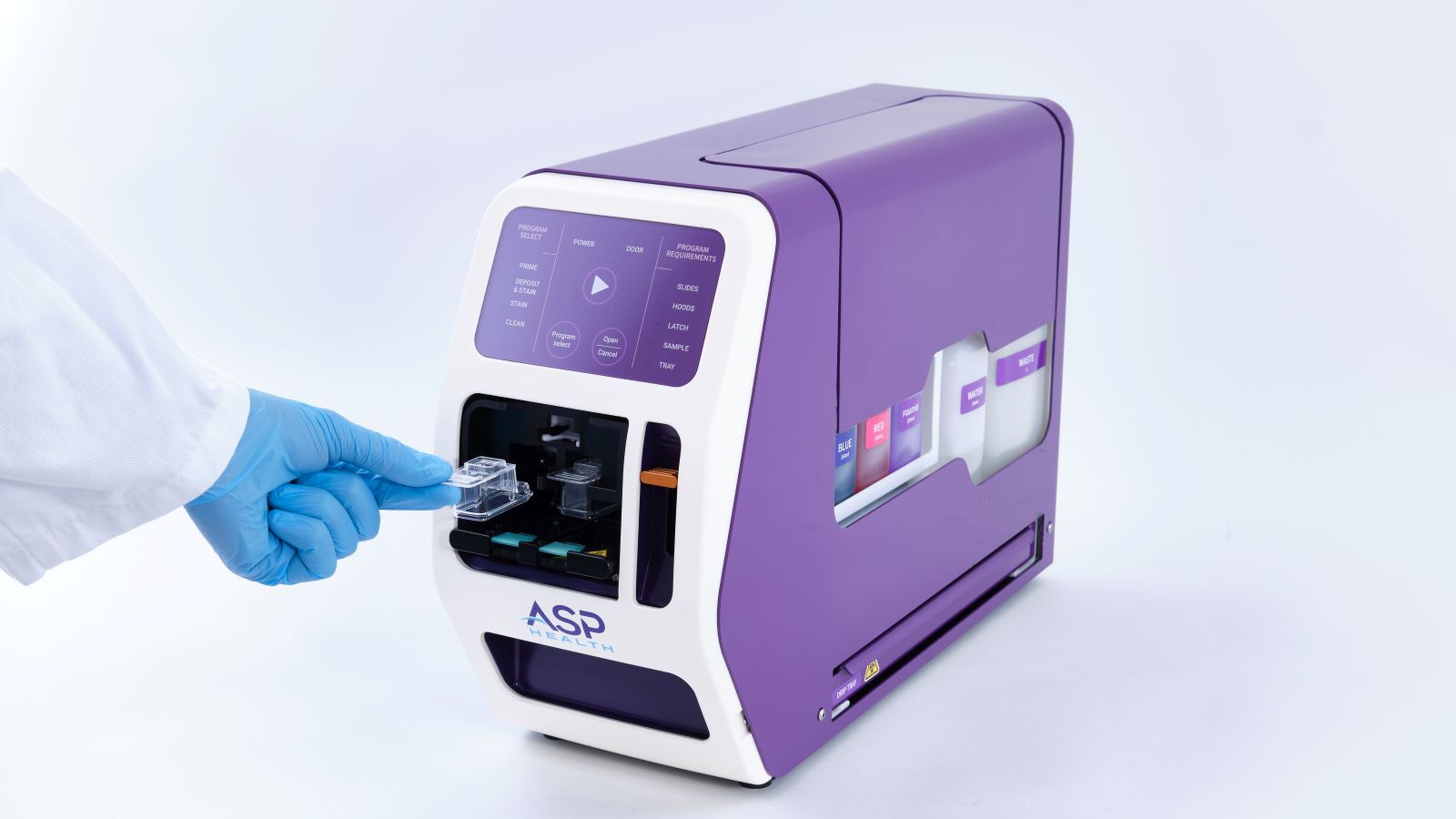
Planet Innovation has partnered with Chicago-based company ASP Health Inc. to help them develop and manufacture an innovative sample preparation instrument to enhance cancer diagnostics through more consistent, high quality patient samples.
ASP Health’s Automated Sample Preparation Technology Platform is transforming the rapid on-site evaluation (ROSE) process for fine needle aspiration (FNA) biopsies, which are most commonly used to diagnose tumors in the thyroid, lungs, pancreas and lymph nodes.
By improving the ROSE process, the platform, which was launched this month, could dramatically reduce the need for repeat invasive FNA biopsies.
“Our goal is to improve cancer survival rates through enhanced diagnosis,” said ASP Health CEO John Hart.
“This platform will make it possible for all facilities that offer FNA procedures to efficiently perform rapid on-site evaluation (ROSE) and provide enriched patient samples for further cancer diagnostic and therapeutic evaluation, leading to improved patient outcomes.
“It’s taken several years to bring this innovative technology to market and Planet Innovation has been a fantastic partner every step of the way.”
Product-market fit program identified problem with the ROSE process for FNAs
ASP Health initially came to Planet Innovation with a novel slide preparation technology. PI conducted a product-market fit program, involving research with leading clinicians, to investigate where ASP’s technology could have the most impact.
The ROSE process associated with FNA biopsies, in particular endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), was identified as an area where ASP Health could make a real difference to patients’ lives.
In an FNA biopsy, doctors use a needle to extract a tissue sample from a tumor, which is then sent to a pathology lab to assess if the tumor is cancerous. For complicated procedures such as EBUS-TBNA, which involves inserting the needle via a bronchoscope, patients are usually placed under general anesthesia.
However, it is often difficult for doctors to know if they have extracted enough sample to enable diagnosis. Depending on the tumor, up to 32% of FNAs must be repeated1 – resulting in delayed diagnosis and the need for patients to undergo a second invasive procedure.
To address this problem, some hospitals and clinics use ROSE to quickly determine if the doctor has obtained a sufficient sample for diagnosis. Depending on the tumor, ROSE can reduce repeat biopsy rates by up to 70%1.
Because the current ROSE procedure is a time-consuming process that requires a cytopathologist or cytotechnologist to manually smear and stain slides while the FNA is performed, it is only used in about 30% of institutions1. This means many patients are having to return to clinics or hospitals to repeat the FNA procedure.
How ASP Health is improving the ROSE process
After the product-market fit program identified the ROSE process as the area to focus on, ASP Health and PI set about designing an instrument that could automate the manual slide preparation process.
Throughout the development process, the PI and ASP Health teams continued to seek feedback from clinicians and cytopathologists around the world to ensure the instrument met their needs.
The resulting platform is fast, efficient and able to deliver consistent, high-quality samples that are equal to, or better than, those prepared manually.
The instrument is lightweight and portable, so it can be placed on a cart and easily moved into operating theatres as needed, with a simple workflow requiring only minimal training to operate.
The patient sample is loaded into a cartridge and inserted into the instrument, which deposits the sample onto two slides – one stained for immediate assessment, the other ready to be sent to the pathology lab if there are enough cells for diagnosis.
The whole process takes under two minutes, and can be quickly repeated if it is found the clinician needs to take another sample.
A strong, collaborative partnership
ASP Health and Planet Innovation worked very closely throughout the entire product development process – from the product-market fit analysis through early prototypes, to building the alpha and beta units, and now ramping up manufacturing for the instrument and consumables.
“The whole process has been very collaborative and very open. It’s feels like we are one team working towards a goal, not a client and a service provider,” John said.
He said the time difference between Chicago and Melbourne had helped to accelerate product development.
“We’ve been able to work on the product almost 24 hours a day; when PI’s Melbourne team finishes their work, our team in Chicago then has the whole day to review and respond, before the Melbourne team picks it up again. This has let us get through the product development process faster and more efficiently than might otherwise have been possible.”
Anthony White, President of PI North America, said he and the whole PI team had relished the opportunity to work on such an innovative instrument from idea through to manufacturing.
“This is the sort of collaborative partnership PI loves. We’ve really enjoyed being able to work closely with a pioneering team helping to productize a novel solution to improve patient outcomes,” Anthony said.
“The ASP platform addresses a real market need. It will make the ROSE procedure more reliable and efficient for hospitals and clinics, thereby improving the diagnosis process for cancer patients.”
The ASP device is in clinical evaluations with products available in early Q1 2022. For more information visit the ASP website.
 Left: A cardboard prototype / Right: Reliability testing at PI
Left: A cardboard prototype / Right: Reliability testing at PI