In London on July 8, 2013, in front of the global IVF community at the European Society of Human Reproduction and Embryology (ESHRE) conference, I presented a paper that revealed the details to the microfluidic pod that enabled automated IVF vitrification for the first time in the world.
Developed by Genea Biomedx and Planet Innovation, the pod is used within the revolutionary automation instrument known as Gavi (Genea Automated Vitrification Instrument). This is a summary of what I presented and highlights that it was the innovation around the consumable ‘pod’ which really held the key to achieving the automation breakthrough.
The vision
During an IVF cycle, embryos which are not transferred immediately back to the patient are placed in cryo storage through a process called vitrification. Since IVF was introduced more than 30 years ago the vitrification process has been very manual. The vision of both Genea Biomedx and Planet Innovation was to reduce variations in IVF vitrification by automating and standardizing the process.
The market wanted a lot
Through extensive voice-of-customer studies we determined that the market had five key requirements from an automated vitrification platform. They were:
- Survival rates as good as the gold standard, which is the Cryotop® open system.
- Regulator authorities wanted a closed system to guarantee no cross-contamination.
- The process had to be automated.
- Multiple embryos had to be vitrified simultaneously.
- Can work on oocytes, cleavage stage and blastocysts.
The biological challenge
The complications in achieving these market needs were that human embryos are fragile, they float, they can change shape and they are very small. This is why the process had remained manual.
Proposed solution
Right from the start, the idea was to secure the embryo in location and exchange the media without damaging or losing the embryo. We didn’t know how we were going to achieve this goal, but we believed that this revolutionary approach held the key.
The problems
The current embryo vitrification process is a manual and specialist task. It involves an embryologist physically moving the embryo from fluid to fluid by hand. As a result the process is highly variable and time consuming. There were also many unknowns in just how to automate the process.
The variables
What was known was that for the automation to be effective and achieve the desired results, six key variables had to be controlled extremely precisely. These were:
- Temperature
- Time
- Volume
- Media concentration
- Speed of fluidics
- Vitrification rate
Manually, it is possible to control one or two of these variables, however, our aim was to control all of them. It initially appeared almost impossible, like trying to hit a bull’s eye from a great distance.
Prototypes
We gathered an extremely talented and diverse team to interpret the voice-of-customer results and develop prototypes. Many concepts were developed and tested through rapid prototyping. Without describing them all, the concepts included a mesh concept, a perforated cone idea, tweezers, a microfluidic cartridge, the golf tee, a wineglass concept and the restricted tube.
Fail often, fail fast
All these concepts ended up being eliminated very quickly. The mesh concept was initially favored as the best idea. However, through testing we discovered that as we drew the solution through the mesh, the sensitive embryos would also be dragged through the mesh and disintegrate. Another failed concept was the restricted tube. With this idea the embryo would be drawn up the tube into a restriction where it was held while fluids were exchanged. However, the embryos would often become stuck in the restriction and could not be retrieved.
The winning idea
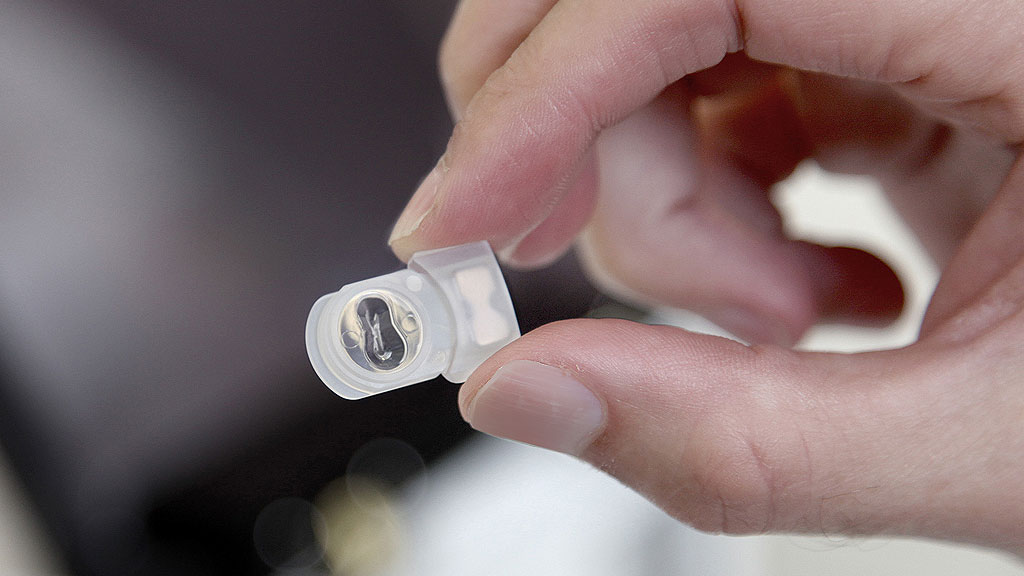
The winning idea was the ‘pod’. We ran through over 15 prototypes as we constantly improved and further improved the concept all the way through to the production design.

The pod was effectively a microfluidic channel that locates and positions the embryo. The vitrification fluidics are allowed to be exchanged with gradual diffusion of the solution around the embryo, which prevents toxic shock on the embryo. Thin wall sections were designed in to the consumable pod to achieve the required vitrification rate and after the final fluid is dispensed the pods are sealed to fully protect the embryo from any liquid nitrogen contamination.
The Gavi instrument controls the embryo microenvironment to within 0.5 degrees Celsius of the protocol temperature. To control the volumes a customized dispensing system was developed to dispense 1 micro liter with 0.2 micro liters accuracy. The instrument also performs the pod sealing in a controlled time, temperature and manner.
We use software to standardise the protocol and control the time, environment, volume and temperature.
All six variables were controlled
The pod, with the Gavi instrument, now controls all six variables: Temperature, Time, Volume, Media Concentration, Speed of fluidics and Vitrification rate. Automated vitrification, which had seemed almost impossible to achieve in 2009, was now a reality.
My two key learnings are:
- Don’t be afraid to take on big challenges. Challenges that people are telling you can’t be solved.
- Secondly, stay true to your big idea but be prepared to learn from failures and change directions until you hit the winning concept. In our case, it was a small, consumable ‘pod’.