Atmo Biosciences, a Planet Innovation early stage venture, has signed a licensing deal with RMIT University securing the exclusive worldwide rights to commercialize a ground-breaking ingestible gas-sensing capsule.
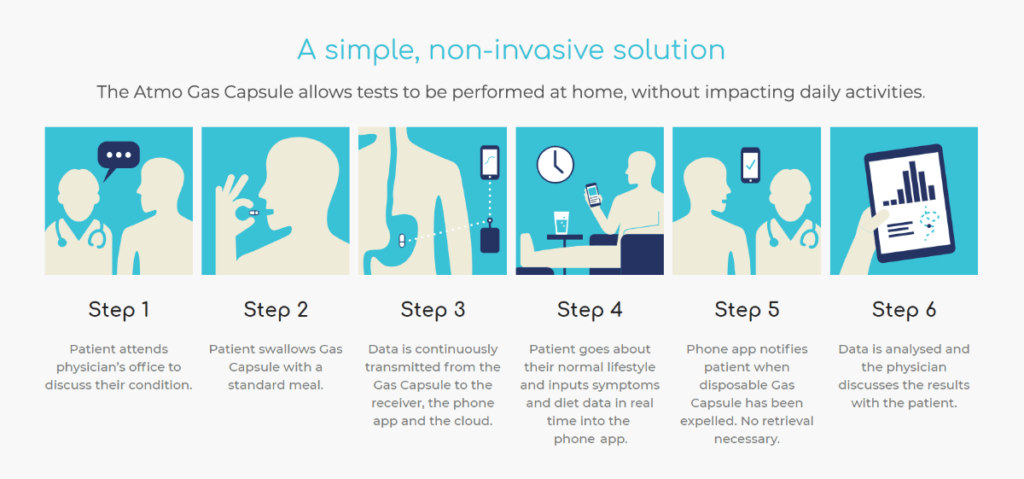
The Atmo Gas Capsule is set to revolutionize how doctors diagnose and treat common gastrointestinal (GI) disorders by offering a non-invasive, low-cost, and more accurate alternative to current technologies.
Patients with gut problems will be able to swallow the capsule – about the size of a vitamin pill – and get real time, continuous detection and measurement of hydrogen, carbon dioxide, methane, and oxygen in the gut. The data can be sent via a handheld receiver and mobile app to the cloud, where it can be analyzed to gain insight into gastrointestinal disorders including small intestinal bacterial overgrowth (SIBO), irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), carbohydrate malabsorption and intolerance.
Atmo Biosciences CEO Mal Hebblewhite said he expected strong demand for the capsule, which is expected to be on the market by 2022.
“One in five people worldwide suffer from a gastrointestinal disorder in their lifetime, and almost a third of these cases remain unresolved, often due to difficulty in diagnosis,” Mal said.
“The Atmo Gas Capsule has the potential to close this gap by offering the ability to identify previously undiagnosed conditions. This will provide relief to the millions of patients who suffer daily without targeted therapy to relieve their symptoms.”
 RMIT Deputy Vice-Chancellor Research and Innovation and Vice-President, Professor Calum Drummond (left), and Atmo CEO Mal Hebblewhite.
RMIT Deputy Vice-Chancellor Research and Innovation and Vice-President, Professor Calum Drummond (left), and Atmo CEO Mal Hebblewhite.
The capsule has already undergone Phase 1 human trials, with results revealing the capsule to be 3,000 times more accurate than breath tests in detecting gas biomarkers.
Capsule co-inventor Dr Kyle Berean, Atmo Chief Technology Officer, said: “We know breath tests suffer from high rates of false positive and false negative diagnoses and we know that gas concentrations in the gut are up to 10,000 times higher than those in the breath. By measuring the hydrogen, carbon dioxide and oxygen produced by the gut directly at the source, our capsule offers vastly more accurate results and unprecedented signal to noise ratios, compared with breath testing.”
The next stage of development at Atmo will include Phase 2 human trials, as well as work to enhance the existing technology and expand the range of gases the capsule can sense. Among other indications, researchers will target biomarkers related to ulcerative colitis, a form of IBD that costs the US healthcare system $US19 billion each year.
Atmo is currently undertaking a Series A capital raise, with significant interest in investment and collaboration from global pharma companies and other corporate and strategic partners.
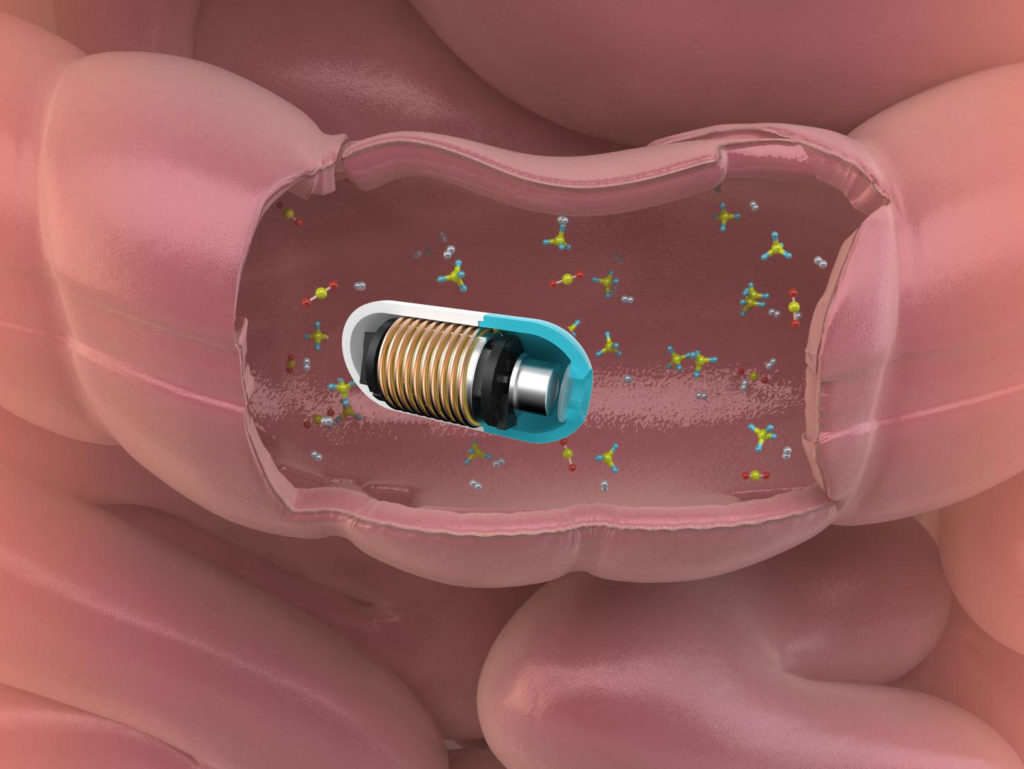 |
What’s inside the capsule?Housed inside a small plastic shell are gas sensors, a temperature sensor, a microcontroller, a radio-frequency transmitter, and silver-oxide batteries. The gas sensors are sealed in a special membrane that allows gas in, while keeping out stomach acid and digestive juices. The capsule relays the data to a small receiver, which in turn transmits it to a phone app and the cloud. |
Find out more about Atmo Biosciences.