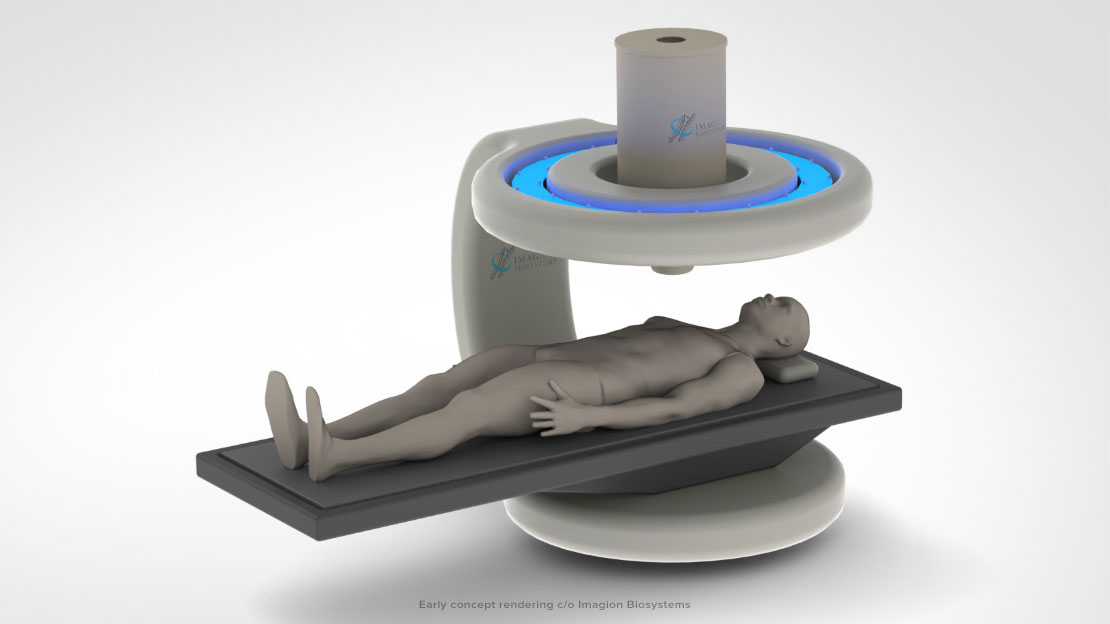
Planet Innovation has entered into a Master Service Agreement with Imagion Biosystems, a company dedicated to improving healthcare through the earlier detection of cancer, to develop Imagion’s MagSense™ instrument technology.
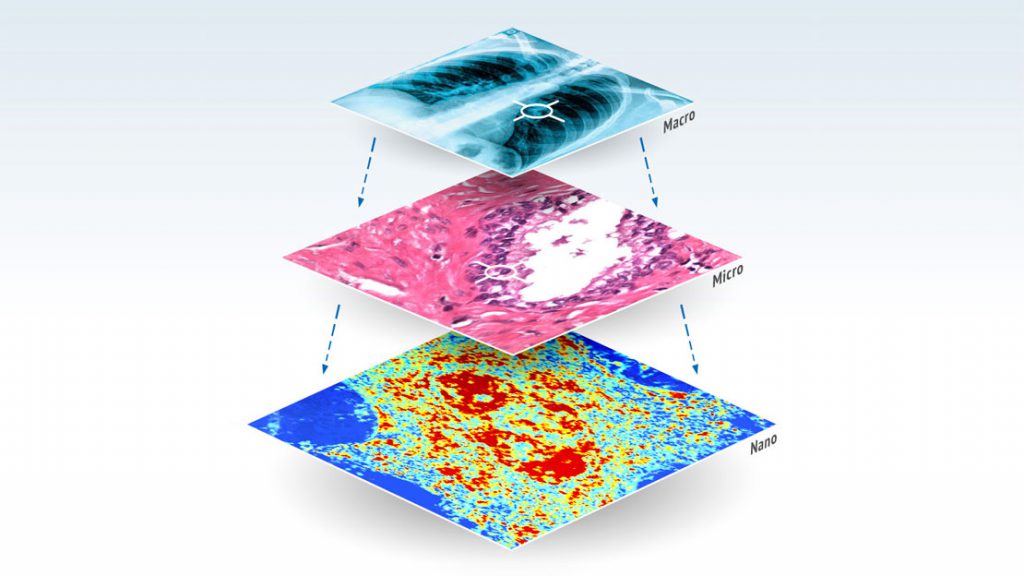
The MagSense™ technology will offer a non-invasive way of detecting specific types of cancerous tumors. Nanoparticles coated with tumor-targeting antibodies are injected into the body, where they circulate through blood flow and bind with targeted tumor cells. The nanoparticles are briefly magnetized by a low-field magnetic pulse, which will allow the MagSense™ instrument’s highly sensitive magnetic field sensors to detect the nanoparticles, and thus the presence of a tumor.
PI and Imagion will work together on design concepts and the development of prototype systems that can be used by Imagion in its key pivotal clinical study for regulatory and commercial clearance.
Bob Proulx, Executive Chairman of Imagion Biosystems, said: “We are very excited to team up with Planet Innovation to assist us in the next steps towards commercialization of the MagSense™ technology. As we are rapidly approaching our first in human study for the nanoparticle component of our technology, we now need to add focus on the instrument platform. Planet Innovation is a world class engineering and design firm that can take our expertise and know-how on the MagSense™ instrument technology to help us develop our first clinical and commercial product.”
Ian Macfarlane, General Manager of PI Design said: “We believe the MagSense™ system can change the way cancer is detected and treated. We look forward to working with the team at Imagion Biosystems to help bring this potentially life-saving technology to market. PI has a strong record of developing and manufacturing innovative diagnostic products and we’ll work closely with Imagion to get this exciting new technology into trials and available to patients in the shortest possible time.”
Imagion has recently reported progress on nanoparticle development for its early feasibility first-in-human study planned for the breast cancer test in the first half of 2019. That study will not require the clinical instrument and will take place following a toxicology study for biological safety of the MagSense™ nanoparticle formulation for human use. Toxicology safety studies are a standard, but very important step, often considered a significant de-risking milestone in pharmaceutical development.