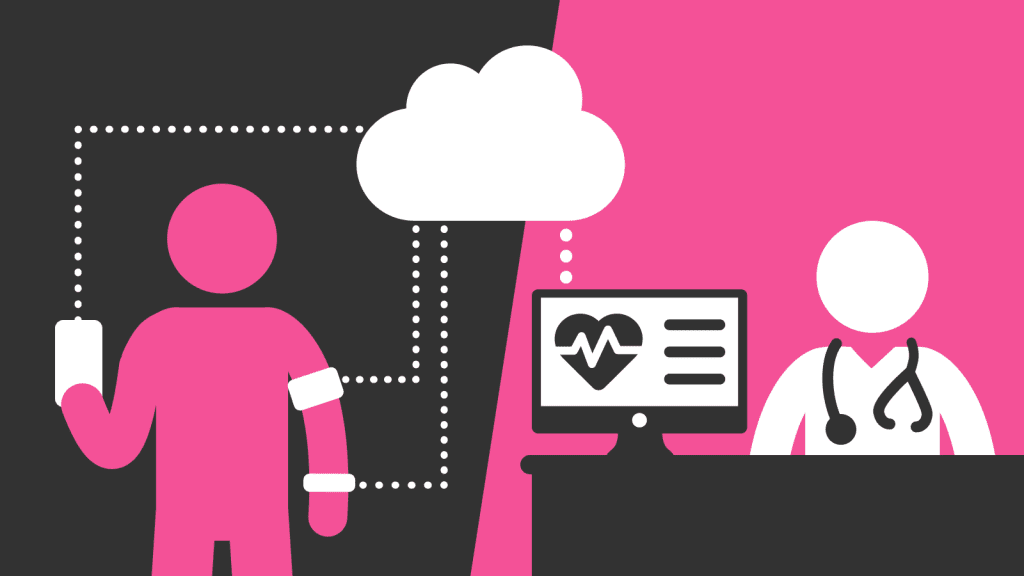
The digital healthcare landscape continues to evolve and a key area that is growing in popularity with both clinicians and patients alike is remote patient monitoring (RPM).
RPM is the use of digital technologies to capture medical data from patients and electronically transmit this information to healthcare providers for assessment and, when necessary, recommendations. For example, this could be monitoring brain activity through a wearable and app, or a cuff that monitors blood pressure.
The global RPM market size is expected to reach USD$16.9 billion by 2030 and is projected to register a compound annual growth rate of 20.2 per cent from 2023 to 20301. Key drivers for this market are the rising prevalence of chronic conditions, an increased geriatric population, new reimbursement codes in the US, and technological advancements.
More and more companies, both new and established, are recognizing the commercial potential of RPM solutions that improve patient care. But creating the software needed to interface between the monitoring device and a cloud-based portal is not a simple task – particularly in regulated healthcare environments where patient privacy and security is paramount.
Using PI’s Software Accelerators for RPM
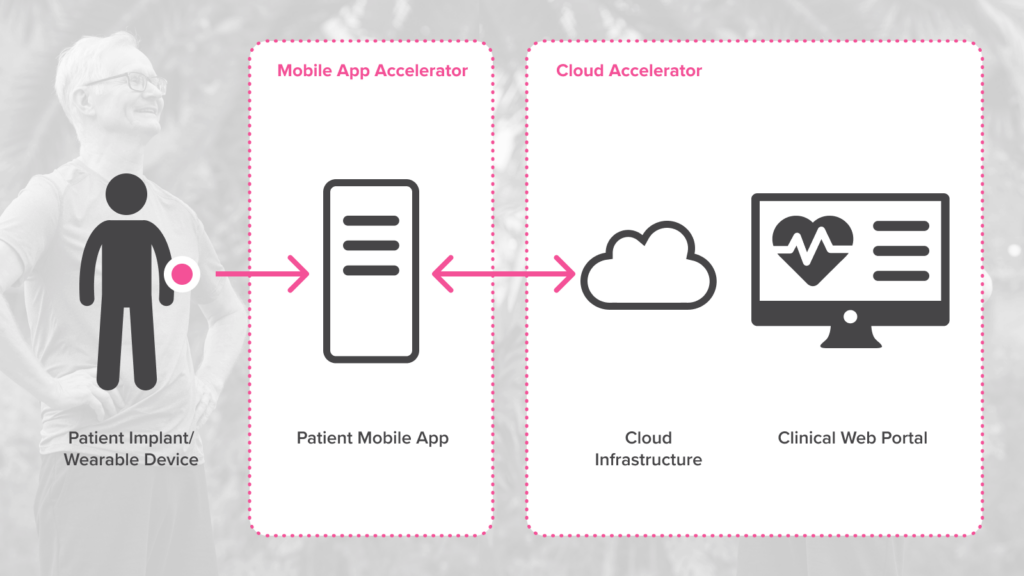
To make it quicker and easier for companies to enter the RPM market, PI has developed Software Accelerators that can be used to build customized software solutions. The accelerators are sets of reusable software components that provide the foundational ‘building blocks’ needed to create secure, regulatory compliant, cloud connected platforms.
For RPM solutions, we use a Cloud Accelerator combined with a Mobile App Accelerator. To develop these accelerators, we drew on our experience creating fit-for-purpose SaMD (software as a medical device) cloud and mobile applications in multiple domains including gut health, brain activity monitoring, respiratory health monitoring and sleep monitoring. This allowed us to develop accelerators with all the components needed to build effective, secure, and regulatory-ready RPM solutions.
Using these tried and tested accelerators as a foundation for your RPM solution reduces development cost and risk, and expedites time to market. It also means you can focus development energy on your product’s unique value proposition rather than the foundational software elements of mobile and cloud connectivity.
How the Mobile App Accelerator enables seamless communication with sensors
The Mobile App Accelerator used in PI’s RPM solution enables seamless communication between a patient device and a companion mobile application – a critical yet highly volatile aspect of medical devices. The accelerator aims to reduce the friction and error rate in Bluetooth communication between patient devices and companion mobile devices on both Android and iOS platforms and provides a set of uniformly designed features to connect and manage a Bluetooth device on Android or iOS.
The Mobile App provides a rapid application development capability which in turn allows our customers to test their product with their potential users for early feedback and product adjustments.
Once the mobile has collected the data (which most of the time, is a test result), we need to upload that data to somewhere – and that’s where cloud connectivity comes into the picture.
Secure and compliant Cloud connectivity with the Cloud Accelerator
The Cloud Accelerator provides secure and compliant storage, processing, and display of patient health data for remote access by healthcare professionals. It supports a set of ready-to-use software components, processes, and best practices for cloud components in a medical device. Like the Mobile App Accelerator, the Cloud Accelerator provides PI customers with cost savings, rapid feature development and reuse of best patterns and practices.
Most importantly, the Cloud Accelerator has cybersecurity and regulatory compliance built in. We see many clients coming to us wanting cloud connectivity solutions, but they haven’t fully considered things like cybersecurity assessment from the device perspective; or HIPAA and GDPR compliance given they are recording patient health information (PHI) as well as personally identifiable information (PII). The Cloud Accelerator has been designed with GDPR and HIPAA compliance in mind to make it easier to achieve compliance with the RPM solution.
Accelerators in practice: Epiminder
A great example of how using accelerators can speed up development is PI’s work with Epiminder. Epiminder is developing an ultra-long term EEG implant that aims to revolutionize the lives of epilepsy sufferers by monitoring seizures. The Epiminder device, which is currently in clinical trials, consists of an implant, a wearable, a patient app and Cloud-based software. The app and cloud software were developed using PI’s Mobile App and Cloud Accelerators. Using the accelerators as a foundation gave us a head start on development – meaning Epiminder were able to start building out requirements almost immediately.
As Epiminder chief executive officer, Rohan Hoare, said: “Using PI’s accelerators meant we hit the ground running and could quickly see progress on features. We look forward to bringing the Epiminder technology to market as soon as possible to improve the lives of those living with epilepsy.”
Summary
With PI’s Cloud and Mobile App Accelerator we’ve built a set of common features that can form the foundation for different RPM solutions. This not only speeds up development time and thus time to market, it also reduces the risks associated with building compliant and secure software. And with the hard work already done to build a compliant platform, clients can focus their energy on building the best product for their customers.
If you would like to learn more about PI’s RPM Accelerator or request a demo, please contact us.
References
- Remote Patient Monitoring System Market Size, Share & Trends Analysis Report by Product (Vital Sign Monitors, Specialized Monitors), by End Use, by Application, and Segment Forecasts, 2023-2030 (researchandmarkets.com)