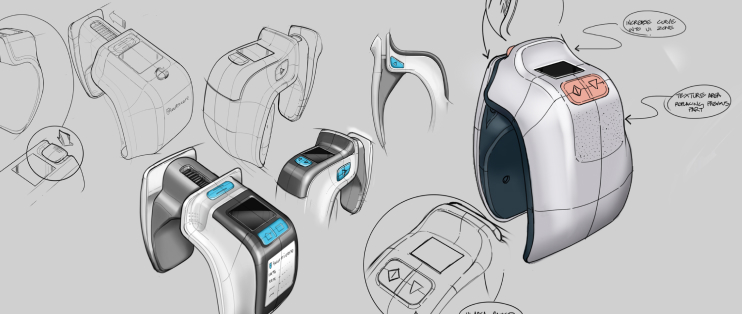
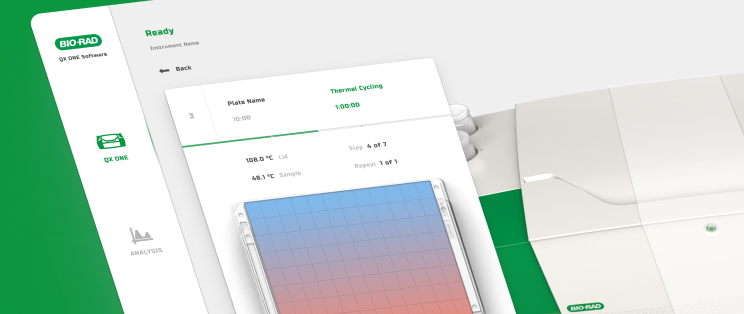
Human Factors & Usability Engineering
We humanize medical technologies through our qualified and expert understanding of people, their behaviors and their limitations.
PI’s internationally-recognized, dedicated team of medical device Human Factors & Usability Engineering (HF&UE) experts are embedded throughout our design and development process, from feasibility to post-market compliance.
We guide design direction – providing insights, backed up by data, on how your system will be used, perform and be safe. We work collaboratively with client, clinical, commercial, systems, regulatory and design teams to understand and provide:
-
- Best-practice use-related assurance for safe, effective, market-leading technologies
- Data for clinical, market and regulatory success
Applied early, our HF&UE team can help minimize any costly reworks and regulatory submission delays, thereby reducing development costs and helping you get into market faster with an optimized system.
Proven
We apply industry best-practice, scientifically robust approaches and methods.
Provable
Our guidance and reports are supported by quantifiable and qualifiable Human Factors data.
Integrated
We guide the design process side-by-side with the development team at the earliest stage to expedite regulatory approval.
Profound
Our clients have found our HFE impact to be profound, providing development focus and assurance that the product or system will be used, and used well.
Grounded in Science
At PI, we know people, their behaviors and their limitations. Our applied Human Factors, Ergonomics and Usability analysis methodologies quantify and qualify use-related safety and user performance; whether it be for on-market advantage and/or regulatory acceptance.
Through the product development lifecycle, we will help you know how your product will perform with intended users and interfacing systems, ahead of design refinement, clinical studies, validation and market release.
Highly Skilled HFE Leaders
Our team has extensive Human Factors, Ergonomics and Usability qualifications, skills, training and experience across most industries, including medical devices and combination products. This includes wide-ranging scope, development stages, technologies, system complexities, and use-related risk, with multi-disciplinary teams.
Our approach is sensible, realistic, and justified. We can guide you to safe, practical, effective, and scientifically supported humanized design solutions.

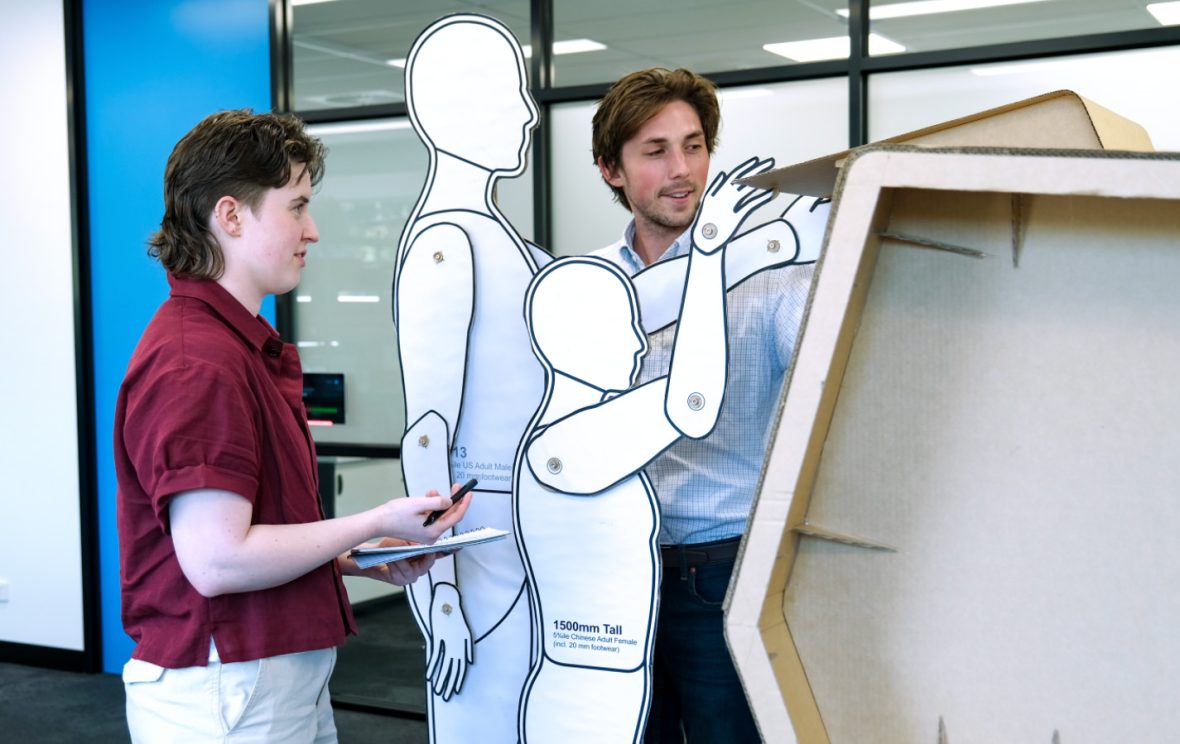
Tailored and Focused Support
With our deep understanding of people and their interactions with system interfaces, we know what matters, when and why. We know where to focus effort to deliver value. From concise human factors analysis through to a complete HF&UE program, we scale our support to meet your commercial and regulatory needs.
During early-stage feasibility, we help you identify the right user requirements and use-related risks to prioritize. This ensures your development effort is focused, and sets your product up for commercial success.
Understanding the “why” to exceed regulatory requirements
We have extensive knowledge of harmonized Human Factors & Usability Engineering regulations, standards and guidance material. Our understanding extends deeper than their contents to the rooted science, their historical development, as well their applicability and application. This allows us to develop and tailor cross-regional regulatory strategies specific to your system, its users, uses and use environments.


Holistic Approach
Our holistic approach to human factors, usability engineering and product development ensures all necessary users, uses and use environments of the product and service ecosystem are sufficiently considered, represented, defined, incorporated, and evaluated in unison.
We bring the financial and efficiency savings of being integrated with the development team, whilst maintaining the impartiality of a third party consultancy.
Working with client, regulatory and internal teams, we help you establish safer and more effective system outcomes to enable optimized uptake and enduring high performance.
Related Capabilities
We take a user-centric and market-focused approach to industrial design, ensuring your products are not only beautiful, but also user-friendly, practical and cost-effective.
Our UI/UX team is experienced in designing user interfaces for a range of applications including device embedded screens, mobile and web apps, and wearables.
The systems team are experienced product developers that specialize in defining a product vision and taking a device on a journey to realize that vision. Our involvement begins at the early stages of product definition and architecture refinement, and continues throughout the development, monitoring risks, performing testing and managing verification activities.