Manufacturing Quality Engineering & Assurance
PI produces the highest quality products that adhere to FDA regulations, crafted within a well-established quality system that undergoes continuous auditing.
These processes take place in certified facilities that meet the standards of ISO 13485:2016 and ISO 9001.
Planet Innovation’s QMS is a crucial framework that ensures compliance with regulatory requirements, drives continuous improvement, and maintains the highest standards of product quality and safety. The system encompasses various processes, procedures, and controls that govern all aspects of the manufacturing process.
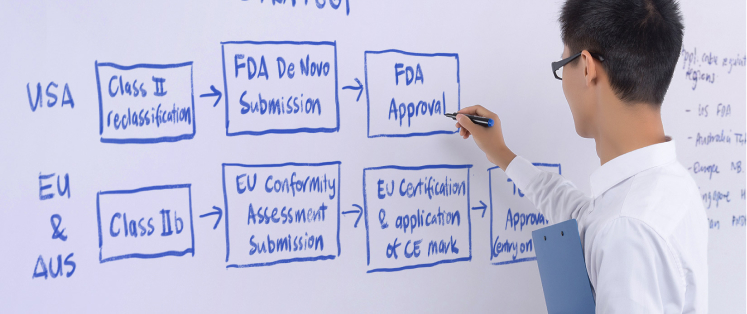
Additionally, our team contains regulatory experts who can assist you in formulating a regulatory strategy to optimize the process of product development, obtaining regulatory approval, and entering the market smoothly.
Quality Policy and objectives
The PI Quality Policy sets the foundation for the entire quality system. Clear quality objectives are established to drive continuous improvement and monitor performance through management review.
Proactive supplier management
Effective supplier management plays a critical role in ensuring the quality and reliability of purchased components and materials. PI conducts evaluations and audits of suppliers, and maintains a strong process for qualifying and monitoring them to uphold high standards of quality and reliability.
Production and process controls
We have clear procedures to govern production processes, including equipment calibration, validation, and maintenance, personnel training, and adherence to appropriate manufacturing and quality standards. In-process inspections, testing, and process validation are carried out to ensure product conformity and consistency.

Structured quality plan for each product
Each product released to manufacturing at PI has a quality plan. This is a comprehensive document that outlines the approach and measures for ensuring quality throughout the product lifecycle. It serves as a roadmap for managing quality activities and maintaining adherence to established standards, regulations, and customer requirements. It provides a structured framework for achieving and sustaining high-quality outcomes.
Corrective and preventative action (CAPA) process
A systematic CAPA process is in place to identify and address non-conformities, deviations, customer complaints, and other quality incidents. This involves root cause analysis, corrective action implementation, and verification of effectiveness to prevent recurrence and drive continuous improvement.
Quality control and testing procedures
Robust quality control measures are implemented throughout the manufacturing process to ensure that products meet specified requirements. This includes incoming material inspection, in-process testing, final product inspection, and release criteria verification.


Record keeping and traceability
Accurate record keeping is essential to demonstrate compliance, traceability, and provide evidence of adherence to the quality system. PI has robust document control procedures utilizing an eQMS to ensure that all necessary documents are properly controlled, reviewed, and updated. This includes standard operating procedures (SOPs), work instructions, complaint records, and other pertinent documentation. We also maintain device history records and batch records.
Experienced and specialized team
PI boasts a team of experts highly skilled across all types of medical device and in vitro diagnostic devices. The team’s key strengths include:
- Validation of Ethylene Oxide Sterilization
- Validation of Radiation Sterilization
- Verification of Packaging
- Commissioning and Maintenance of Controlled Environments
- Biocompatibility Expertise
- Proficiency in Statistical Analysis
- Equipment Qualification including Installation, Operational and Performance Qualification (IQ/OQ/PQ)
- Six Sigma Mastery
- Expertise in Lean Manufacturing
- Certified Lead Auditors
Continuous improvement mindset
A culture of continuous improvement is fostered within the organization through the use of quality metrics, management review meetings, internal audits, and feedback mechanisms. Lessons learned and best practices are shared and incorporated to drive ongoing enhancement of the quality system.
Collaboration with global quality management systems
PI collaborates with clients across the globe, gaining experience with diverse quality management systems, certifying agencies, and regulatory bodies such as BSI, TUVRheinland, UL Solutions. We undergo regular audits alongside our clients and their notified bodies to fulfill obligations, ensure compliance, and establish ourselves as an essential design and manufacturing partner. This process enhances our quality systems, ultimately benefiting all our clients.
Related Capabilities
At our FDA registered and ISO 13485:2016 compliant manufacturing facilities, we can produce anything from low-complexity devices to large floor-standing in vitro and clinical chemistry instruments, in quantities ranging from small batches to high-volume builds.
PI’s quality team are experienced in quality design assurance for regulated and non-regulated medical devices. Our robust quality management system is certified to ISO 13485:2016. We also have regulatory experts who can provide tailored regulatory advice for developing medical products.
Our New Product Introduction team are experts on how to manufacture high quality, regulated healthtech products so they can be brought to market quickly and cost effectively.