
Design Quality Assurance & Regulatory Affairs
A flexible quality management system and tailored regulatory advice for developing medical products.
PI’s quality team are experienced in quality design assurance for regulated and non-regulated medical devices. Our organization has implemented a robust quality management system that holds certification to ISO 13485:2016 from BSI. This system encompasses the necessary processes for the commercialization of medical devices/in vitro diagnostic devices, laboratory/research equipment and cGMP equipment.
Additionally, our team contains regulatory experts who can assist you in formulating a regulatory strategy to optimize the process of product development, obtaining regulatory approval, and entering the market smoothly.
Gated product development process
PI’s Product Development Process (PDP) follows the fundamental FDA and ISO 13485 phase gated process. We tailor each program for every client to ensure the best possible product is developed to meet their individual business case and commercial objectives.
A Design and Development Plan (DDP) is used for each project. The DDP is the detailed plan that defines the development process tailored for each project.

ISO 13485 certified Medical Device/IVD design process
The PI design assurance team collaborates closely with design engineers to guide products through the design control process. Our highly skilled PI design assurance engineers specialize in design control and risk management. They work in coordination with both our internal regulatory affairs team and our clients’ regulatory affairs team to ensure that the product is prepared for submission once the design output stage is completed.
ISO 9001 QMS for equipment intended for research use
The PI ISO 9001 Quality Management System (QMS) is typically employed for laboratory or research use equipment when the stricter ISO 13485 system is not required for regulatory compliance. Customized quality plans are created to optimize the development process and guarantee the suitability of the product for its intended use upon completion of the design control process.


Experts in producing cGMP equipment
Clients seeking PI’s expertise in developing equipment intended for cGMP manufacturing work closely with leaders in the quality team to define the ultimate purpose of the product, along with the necessary deliverables and controls tailored to their unique requirements. These products undergo fully customized design and quality plans to ensure adherence to client specifications.
This is important because the development of equipment intended for cGMP manufacturing lacks a standardized requirement, as the primary responsibility rests with the drug and/or biologic manufacturer.
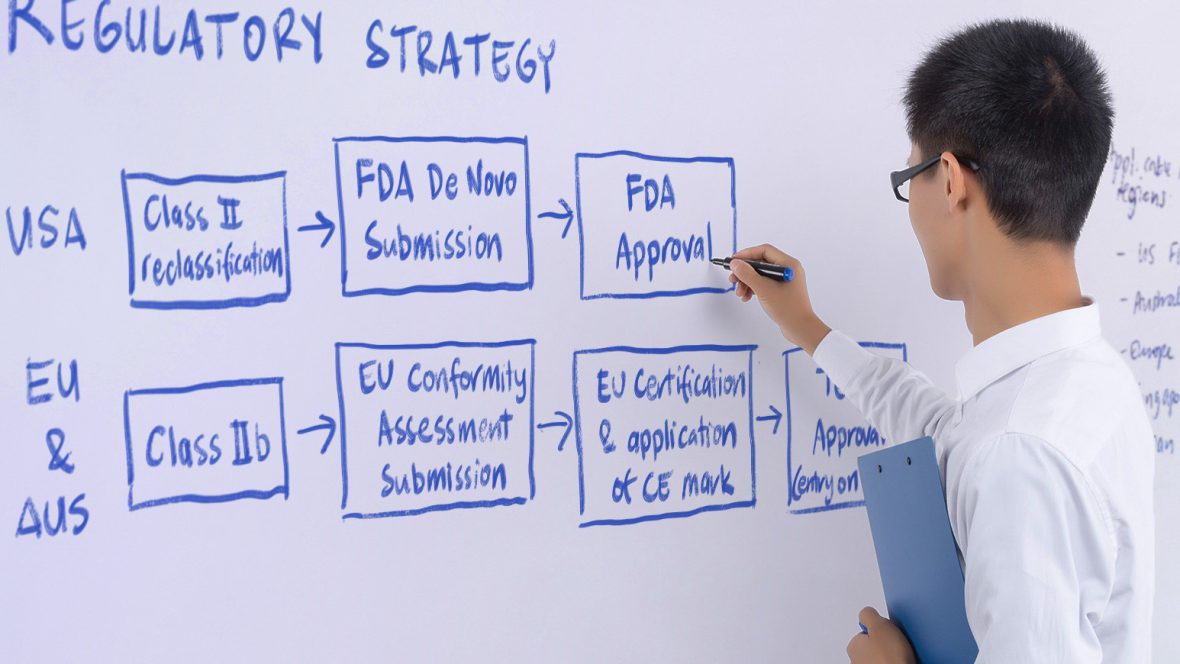
Regulatory Strategy and Advice
By providing strategic guidance and integrating regulatory considerations into the product development process, our regulatory team contributes to the development of more mature regulated products. Our team of regulatory experts, clinical affairs specialists, and product development professionals is backed by a well-established Quality Management System, ensuring comprehensive support throughout the regulatory journey.
The services provided by our regulatory team include:
- Defining the regulatory pathway for the desired jurisdictions
- Developing a comprehensive regulatory strategy
- Designing and executing verification testing documentation to ensure the documents are “submission ready” at the end
- Assisting with investigational testing and device license applications
- Facilitating clinical trial design
- Compiling technical files and preparing regulatory submissions
- Providing support during regulatory reviews
Video Series
Krystal Santiago, VP Regulatory Affairs at PI, discusses ways to work with the FDA when it comes to developing a new, innovative product.
Krystal Santiago explains how you can save time and minimize risks by planning your regulatory strategy early.
Krystal Santiago discusses the shift towards point-of-care diagnostics and its drive to reduce test times while meeting stringent regulatory standards.
Quality System Creation and Quality System Audit
If your organization has not yet obtained certification for the design, development, manufacture, and delivery of your medical device/IVD, or requires assistance with the FDA approval process, PI can tailor a customized solution for you. We can provide training and support to guide you through the ISO 13485 certification audit successfully.
PI employs certified quality system auditors who are available to support and conduct audits of your Quality Management System (QMS).
Related Capabilities
PI has experts across all aspects of product commercialization from initial product definition to achieving market success. We start with in-depth market analysis and MVP definition, then follow through with actionable plans covering regulatory compliance, clinical development, marketing and product launch.
PI produces the highest quality products that adhere to FDA regulations, crafted within a well-established quality system that undergoes continuous auditing. These processes take place in certified facilities that meet the standards of ISO 13485:2016 and ISO 9001.
Our New Product Introduction team are experts on how to manufacture high quality, regulated healthtech products so they can be brought to market quickly and cost effectively.


