
Commercial Services
Providing business guidance and expertise from idea through to product launch, to help ensure commercial success.
PI has experts across all aspects of product commercialization from initial product definition to achieving market success. We start with in-depth market analysis and MVP definition, ensuring the product concept is robust and market-aligned. As the journey progresses, our focus shifts to optimizing design, ensuring regulatory compliance, and crafting effective marketing strategies. This comprehensive approach ensures that the product not only meets market standards but also stands out in competitive landscapes.
Commercial Services
Regulated health experts.
Every member of the PI team brings deep healthcare experience, allowing us to provide specialist and tailored support in developing commercial strategies for regulated products. From capital raising to commercial launch, we help you navigate the complex process of commercializing a regulated healthcare product.
Our services include:
- Business Strategy & Advice
- Product Definition
- Manufacturing Readiness Review
- Regulatory Strategy & Advice
- Clinical Development
- Branding & Product Marketing

Innovation workshops
Our PI Innovation Workshops are designed to support clients at various stages of their product’s journey. These workshops offer tailored guidance on market exploration, product development strategies, and the nuances of market entry and expansion. By employing PI’s structured Innovation process, we address the evolving challenges and opportunities at each stage, ensuring a cohesive and strategic approach to achieving and sustaining market success.
Business Strategy & Advice
We’ll help you develop business strategies and materials to support capital raising or grant submissions. We can also advise on the most appropriate business model and path to market, so you can build a commercially successful business.


Product Definition
In this critical phase, we work closely with you to clearly define your product’s unique value proposition, target market, and user requirements. This process involves deep market analysis and customer insights to ensure that your product meets a genuine market need and stands out from the competition. Our goal is to refine your product concept into a compelling, market-ready solution.
Manufacturing Readiness Review
Before moving to full-scale production, our team conducts a comprehensive Manufacturing Readiness Review to ensure that all aspects of the product design are optimized for manufacturing efficiency, quality, and cost-effectiveness. This review covers supply chain management, production processes, quality control, and scalability planning, ensuring a smooth transition from prototype to production.

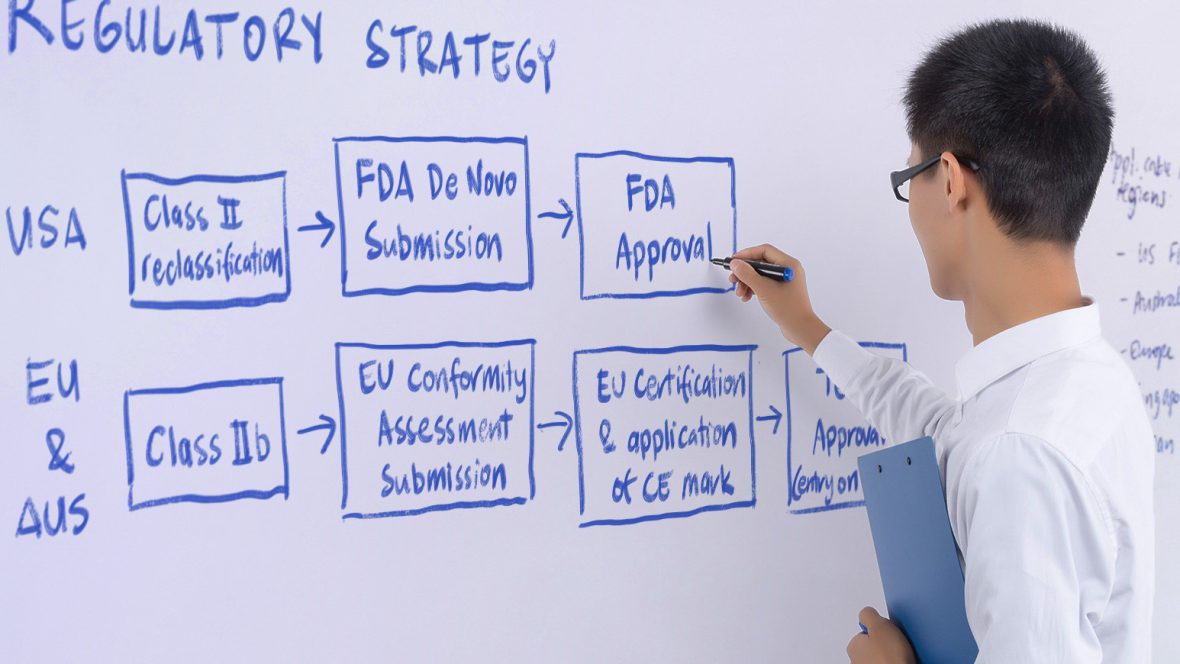
Regulatory Strategy & Advice
We’ll work with you to develop a regulatory strategy to streamline product development, regulatory approval and market entry. This includes identifying the best path to market and aligning marketing claims, product design, and verification and validation activities.
Clinical Development
We’ll help you arrange clinical trials for regulatory approval, and to determine the safety and efficacy of a solution. We’ll work with you to develop the protocols needed to gain ethics approval, and can also provide support with finding study sites.


Branding & Product Marketing
Our team of marketing experts can help you develop strong brand positioning and identity. We can work with you to develop key marketing, user and training materials to proactively prepare for product launch and post-launch support.
Contact Us
Talk to us about how we can help you commercialize your medtech product.
Head into the product development phase feeling confident you are developing the right product for the right market segment, designed to meet customer needs.
Contact us